EU Horizon Projects

Altertox serves as a Communication and Dissemination (C&D) Work Package leader in EU research projects, providing a full range of services to enhance visibility and impact.
Our C&D activities include:
- Strategic Plan
- Stakeholder Mapping
- Web Presence
- Newsletter Management
- Webinars, Conferences, and Training
- Audiovisual Content
- Event Organization
- Media Outreach
- EU Policy/Lawmakers
- Outreach
- KPIs & Surveys
our current eu research projects

PrecisionTox
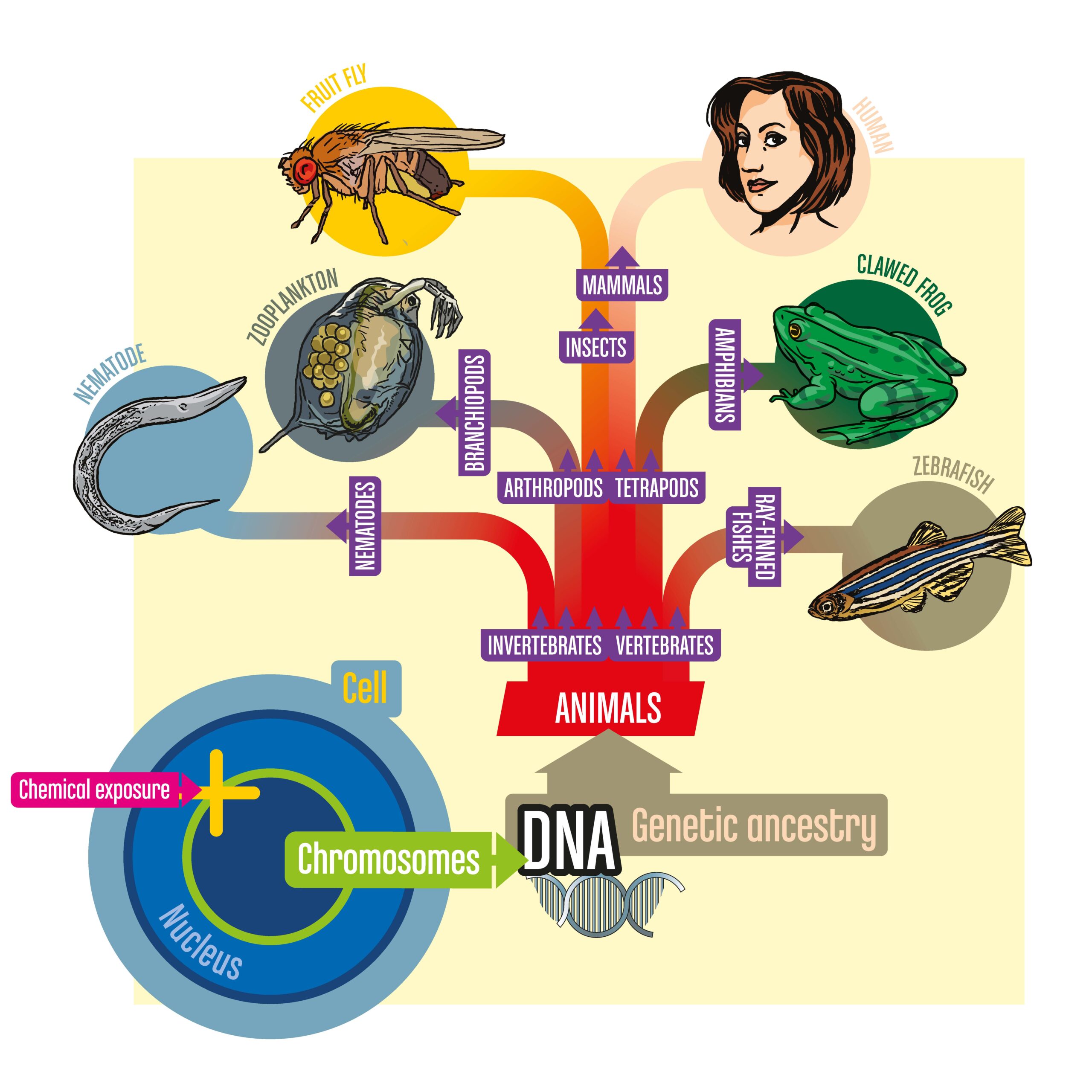
The project aims to enhance chemical safety assessments by using biomedical research species (e.g. Drosophila melanogaster, C. Elegans, Daphnia Magna…) integrating diverse fields of knowledge such as evolutionary biology, and applying advanced computational methods to determine which chemicals are toxic using omics and grouping.

ONTOX
The ONTOX consortium aims to develop a functional and sustainable approach to advancing human risk assessment of chemicals without using animals, aligning with the principles of 21st-century toxicity testing, next-generation risk assessment (NGRA) and probabilistic risk assessment.

Panoramix
Bringing together 11 partners, this project aims to develop new tools for assessing the risks of complex real-life chemical mixtures to protect European citizens and the environment looking at endocrine disrupting substances.

NAMWISE
This project aims to promote the adoption of New Approach Methodologies (NAMs) by providing concrete guidance, recommendations, and training resources for the effective validation, integration, and use of NAMs in assessing the safety and efficacy of chemicals and pharmaceuticals.



Budget €19 305 583,75
Coordinator: University of Birmingham, United Kingdom
C&D actions examples




📅 starts 1 May 2021
📅 ends 30 April 2026
🇪🇺 Grant Agreement: 963845
Budget €17 211 050
Coordinator Vrije Universiteit Brussel, Belgium
C&D actions examples
📅 starts 1 November 2021
📅 ends 31 October 2025
🇪🇺 Grant Agreement: 101036631
Budget € 4 471 092,50
Coordinator Danmarks Tekniske Universitet, Denmark
C&D actions examples
📅 starts December 2024
📅 ends May 2027
🇪🇺 Grant Agreement: 101191595
Budget € 2 049 890,00
Coordinator Ineris,
France
C&D actions examples

Discover more about our communication and dissemination expertise:
Contact Us
If you are interested in working with us – just send a message