Life Sciences Impact

We’re not just communicators: we’re a powerhouse team of experts in science, toxicology, policy, and communication.
This unique mix means we get the complexities of your field and craft precise, impactful stakeholder mapping, audience targeting and communication & dissemination (C&D) strategies that fit your project like a glove.
We also know that C&D isn’t one-size-fits-all. Time, budget, obligations, and preferences vary—that’s why our offer is fully customizable and on-demand.
Choose the level of actions, channels, and creativity that fits your needs, and we’ll make it happen.
You bear it → Your project gets the visibility it deserves.
You like it → Your project grows sustainably and thrives.
You love it → Your project connects, engages, and leaves a lasting impact.
More tailoring. More precision. More creativity. Let’s build the perfect C&D strategy for you.
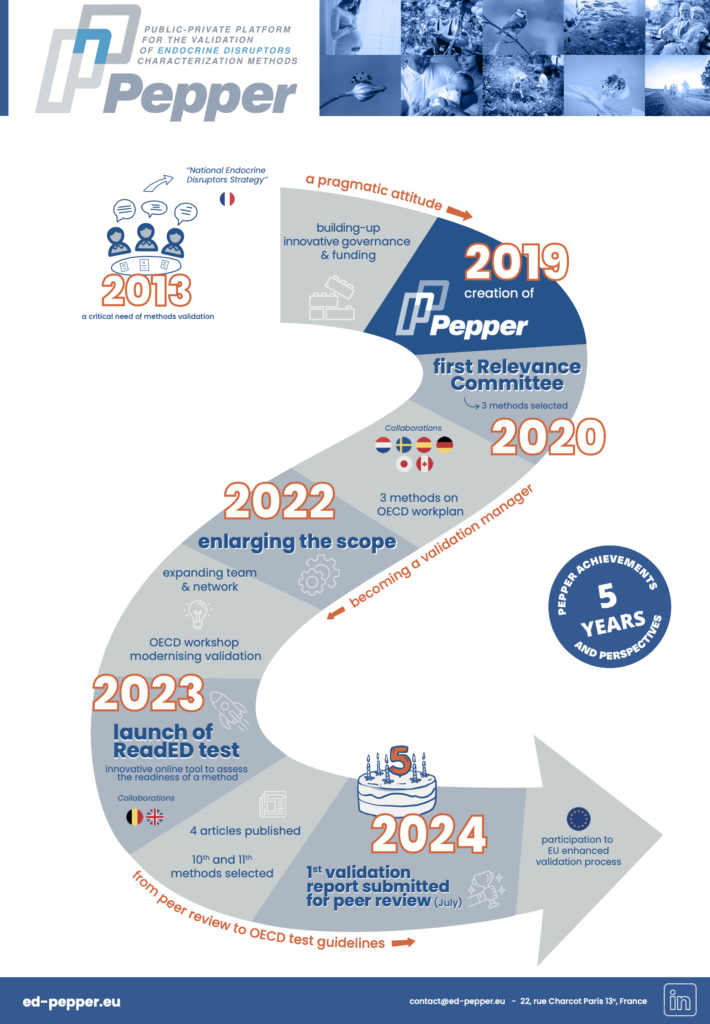
You bear with it
You like it
You love it
- Analysis > Strategy
- Logo, templates (visual identity)
- Website creation & updates
- Newsletters
- Social media creation & updates
- Webinars
- Identifying key persons/organisations
- Analysis > Strategy
- Logo, templates (visual identity)
- Website creation & updates
- Newsletters
- Social media creation & updates
- Webinars
- Identifying key persons/organisations
- Events organisation (e.g. trainings, workshops)
- Video tutorials
- Press releases
- Goodies
- Podcast
- Analysis > Strategy
- Logo, templates (visual identity)
- Website creation & updates
- Newsletters
- Social media creation & updates
- Webinars
- Identifying key persons/organisations
- Events organisation (e.g. trainings, workshops)
- Video tutorials
- Press releases
- Goodies
- Podcast
- Video animations
- Video interviews
- Press articles
- Policy events
- General public dissemination
Contact Us
If you are interested in working with us – just send a message